What is a Rapid Review?
Rapid reviews can provide rigorous, expedited data on COVID-19 policy outcomes

When looking for the answer to a question about COVID-19, the amount of evidence to sift through is considerable. For example, ‘LitCovid’ is a curated literature hub for tracking up-to-date scientific information about the novel Coronavirus. It is the most comprehensive resource on the subject, providing currently central access to over 100,000 (and growing) relevant articles.
However, there has been a tendency for single research studies to be used to inform the public. And when these studies find their way into the mainstream media, they are often not set against the existing evidence. This can lead to erroneous conclusions, particularly when available evidence – that may or may not be contrasting – answering the same question is overlooked.
Systematic reviews aim to overcome the ‘cherry-picking’ of studies by assessing all available evidence for a particular question. They are designed to identify and critically appraise all the available evidence for a particular question and synthesise the data to provide a meaningful summary and are the mainstay for informing clinical decisions and healthcare policy.
‘A good-quality, thorough systematic review, which we shall call a full review, can take several months or even years to complete. According to one estimate, the median time taken is 61 (IQR 33–87) weeks. For this reason, in recent years, because of insistent demands for information, methods for producing systematic reviews more rapidly have been proposed, and it has been suggested that in such cases it should be possible to complete a review within eight weeks. Reviews of this kind, in which the processes involved are simplified, can be achieved with fewer resources and in a shorter time-frame and are therefore appealing, particularly to policymakers.‘
Aronson, Heneghan et al. (1)
Full systematic reviews require considerable resources, and as the evidence moves on, they can often be out of date by the time of publication. This has mainly been the case for covid research, which has evolved at a considerable pace. Also, the study question may have lost relevance.
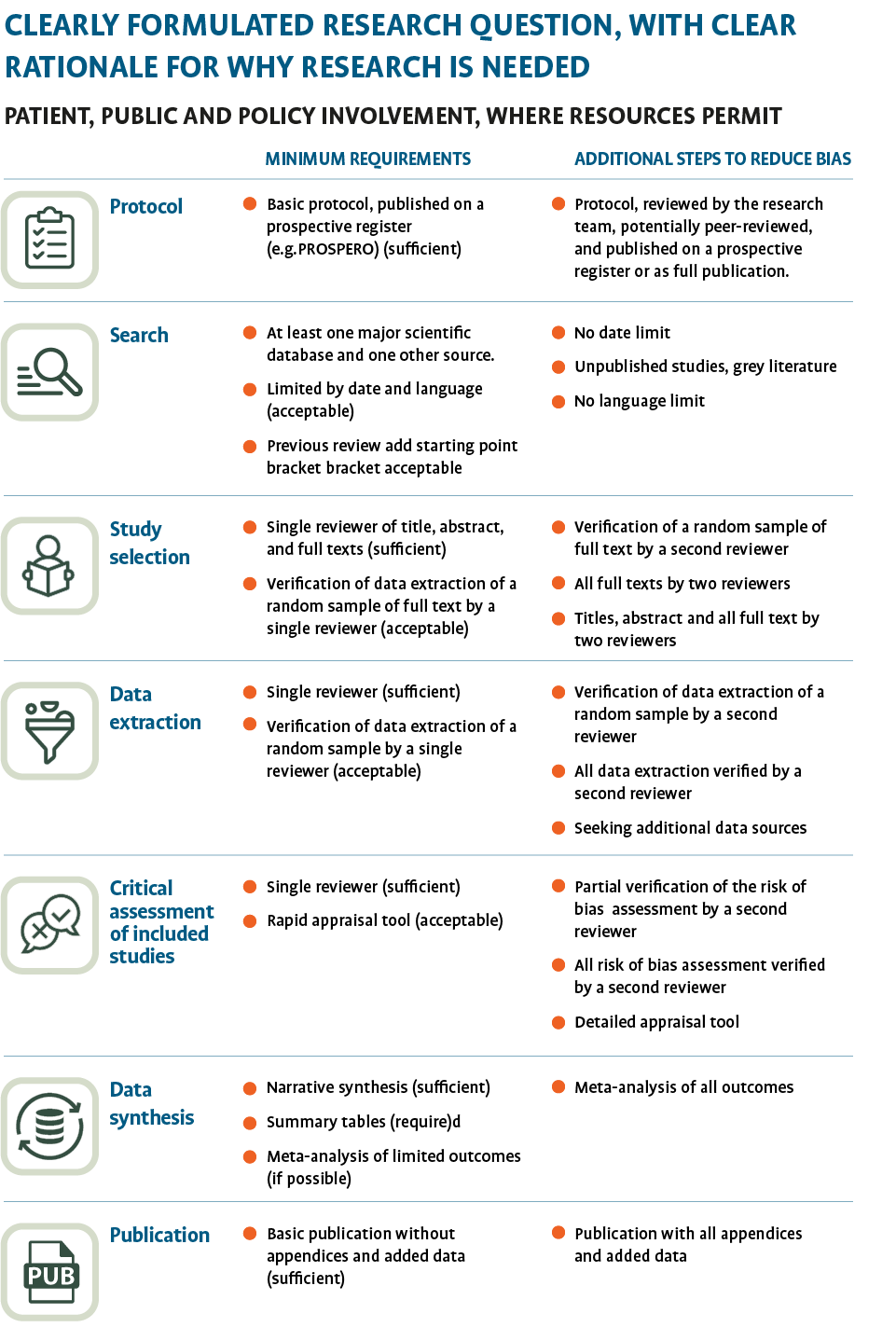
One approach to improve the timeliness is to omit some steps in the review process that speed up publication without affecting the review quality and the confidence in the results. We use a framework that accounts for the available time, resources and the need for expediency but includes those requirements that add rigour to systematic reviews (See figure 1). (2)
The Collateral Global reviews include a clear question; a search strategy that includes at least one major scientific database (LitCovid) and one other source (e.g., searching bibliographies of retrieved articles) alongside several steps to extract study information, assess quality (3) and synthesize the data from the studies to facilitate timely publication.
The reviews produce a thorough answer that can be used by policymakers to inform and enhance decisions. We also use the reviews to assess whether a full systematic review or updates are required. Finally, we draw conclusions for policy, practice and for patients based on the evidence.
Where to find the COVID-19 evidence
The following databases provide up to date resources for finding research evidence on COVID-19.
- WHO COVID-19 Database: The global literature cited in the WHO COVID-19 database is updated daily (Monday through Friday) from searches of bibliographic databases, hand searching, and the addition of other expert-referred scientific articles.
- LitCovid: A curated literature hub for tracking up-to-date scientific information about the 2019 novel Coronavirus. It is a comprehensive resource on the subject, providing a central access to relevant articles in PubMed.
- medRxiv: A free online archive and distribution server for complete but unpublished manuscripts (preprints) in the medical, clinical, and related health sciences.
- Google Scholar: Provides a broad search for scholarly literature across many disciplines and sources: articles, theses, books, abstracts and court opinions, from academic publishers, professional societies, online repositories, universities and other web sites.
- Trip Database: Trip is a clinical search engine designed to allow users to quickly and easily find and use high-quality research evidence to inform their practice and/or care. Trip includes grey literature such as government documents that are vital to our understanding.
Oxford Centre for Evidence-Based Medicine Levels of Evidence
The quality of included studies is assessed using an adapted version of the Oxford Centre for Evidence-Based Medicine Levels of Evidence. For this review, included studies will be ranked as:
- Level 1: a systematic review;
- Level 2: non-systematic review, individual cohort study, retrospective cohort study or “outcomes” research or ecological studies;
- Level 3: individual case-control study;
- Level 4: case-series; and
- Level 5: expert opinion without an explicit critical appraisal, or based on “first principles”.
Endnotes
- Aronson JK, Heneghan C, Mahtani KR, et al. A word about evidence: ‘rapid reviews’ or ‘restricted reviews’? BMJ Evidence-Based Medicine 2018;23:204-205.
- Plüddemann A, Aronson JK, Onakpoya I, et al. Redefining rapid reviews: a flexible framework for restricted systematic reviews BMJ Evidence-Based Medicine 2018;23:201-203.
- Oxford CEBM Levels of Evidence to assess study quality. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
